What you should know about salicylic acid (sal-i-SILL-ic)
Salicylic acid as a medication is most commonly used to help remove the outer layer of the skin with skin conditions such as acne, warts, calluses, corns, psoriasis, seborrhea dermatitis, dandruff, ringworm, and ichthyosis. Salicylic acid and acetylsalicylic acid (commonly known as aspirin) are derived from salicin, originally extracted from the bark of the white willow (Salix alba) tree.
Technically, salicylic acid is classified as a keratolytic, or peeling agent, and works by causing the outer layer of skin to shed. It is dispensed in both prescription and over-the-counter (OTC) medications as an active ingredient in creams, gels, lotions, ointments, pads, plasters, shampoos, soaps, and topical solutions.
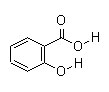
Salicylic acid is a beta hydroxy acid — an organic compound that has a carboxylic acid functional group and hydroxy functional group separated by two carbon atoms. In cosmetics, the term beta hydroxy acid refers specifically to salicylic acid.
Synonyms: 2-hydroxybenzoic acid/salicylic acid; salicylic acid (Tech.); salicylic acid (sublimed); salicylic acid (Pharm.); salicylic acid extra pure; salicylic acid gr; 2-hydroxybenzoic acid
Molecular Formula: C7H6O3
Molecular Weight: 138.12
Melting point (Mp): 158–161 °C
Salicylic acid has been used medicinally throughout most of recorded history. The Egyptians used an infusion of myrtle leaves to relieve back pain. Hippocrates, the famous Greek physician, prescribed willow leaves and bark to reduce fever and pain, including labor pains. In Roman times, both the statesman Pliny the Elder and the alchemist/physician Galen wrote about the use of willow leaves. Native North Americans are said to have made salicylic pain remedies from birch bark. Interestingly, willow bark was reserved for wicker-making in medieval Europe, and its medicinal use was prohibited.
The US Food & Drug Administration (FDA) has approved salicylic acid at various strengths (e.g percentages) as an active ingredient in OTC medication for:
- acne (blemishes, pimples, whiteheads, blackheads)
- antifungal agent (due to its ability to promote exfoliation)
- corn/callus remover (as a collodoin-like vehicle or plaster vehicle)
- dandruff
- psoriasis
- seborrheic dermatitis
- external analgesic (relieve pain) for poison ivy/oak/sumac
- skin protectant for poison ivy/oak/sumac, wart remover
Proper use of salicylic acid depends upon the form of the medication — always read the label instructions fully. Unless directed by a physician, abrasive soaps, cleaners, alcohol-containing preparations, topical preparations containing peeling agents, cosmetics or soaps that dry the skin, medicated cosmetics, and other topical skin medicines should not be used on the same affected areas treated by salicylic acid.
Topical salicylic acid may be absorbed through the skin. Animal studies have shown that salicylic acid can cause birth defects when given orally in doses about six times higher than the highest dose recommended for topical use in humans. Topical salicylic acid has not been reported to cause problems in nursing babies. Young children may be at increased risk of skin irritation and unwanted effects because of increased absorption through the skin. Salicylic acid should not be applied to large areas of the body, used for prolonged periods of time or used under airtight bandages. Use of salicylic acid may also increase the free drug concentrations of other drugs, including hypoglycemics, anticoagulants and methotrexate.
FDA regulations specify allowable dosages and concentrations of salicylic acid. It is imperative that medications containing it are used only as directed. Topical use may cause allergic contact dermatitis as well as excessive drying and irritation of the skin. Some people, especially asthmatics, are highly sensitive to salicylates. Because of absorption through the skin, overdose can result in salicylate poisoning in rare cases.
DermaHarmony makes dermatologist recommended salicylic acid soaps and shampoo bars. Our National Drug Code (NDC) Labeler Code is 71819. We make both OTC and non-OTC salicylic acid products.